
Clearum™ HS Dialyzer
There is a difficult balance between removing toxins while retaining critical proteins. The Clearum™ HS high flux dialyzer can do both.1,5,6 Its performance is coupled with a BPA free design8 and reduced environmental impact,2-4 making it your dialyzer of choice.
The membrane that matters: safe,1 effective,1 green2-4,14
Clearum™ high flux steam sterilized (HS) dialyzers are standard hemodialysis dialyzers with a biocompatible membrane that provides adequate balance between diffusion and convection in HD, HF and HDF.1,5,6 Additionally, they have demonstrated usability on many machines on the market.7
Learn more
Safe
- BPA free8 : BPA is a highly protein bound toxin that is difficult to remove during dialysis. The Clearum™ HS dialyzer avoids this additional protein bound toxin9
- Steam sterilization improves hemocompatibility10-12
Effective
- Effective blood purification without critical protein loss1,5,6
- Based on the sieving coefficient: retains essential nutrients and proteins1,5,6
Green
- Made of polypropylene: reduces final product weight14 and reduces carbon footprint by 60%2
- Steam sterilized: avoids releasing ozone material into the environment4
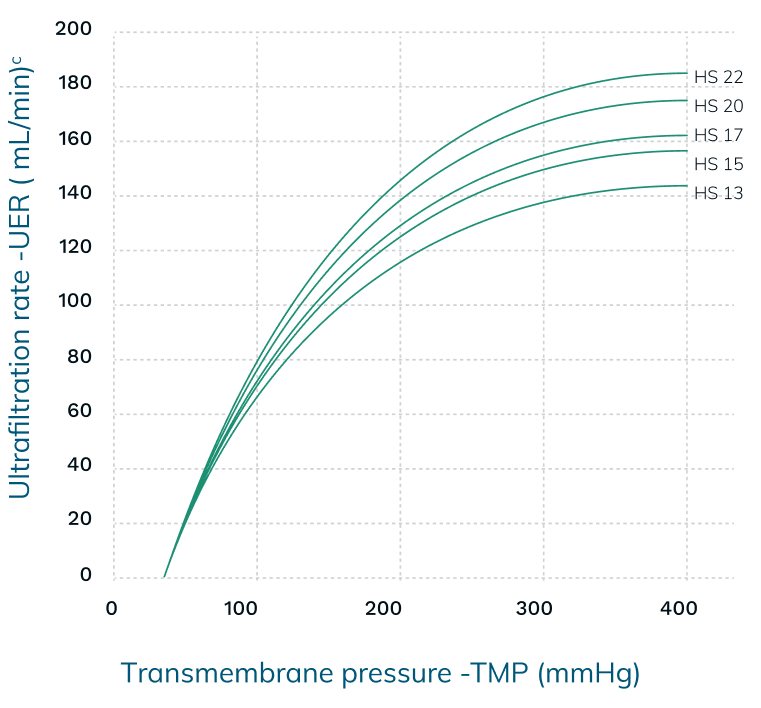
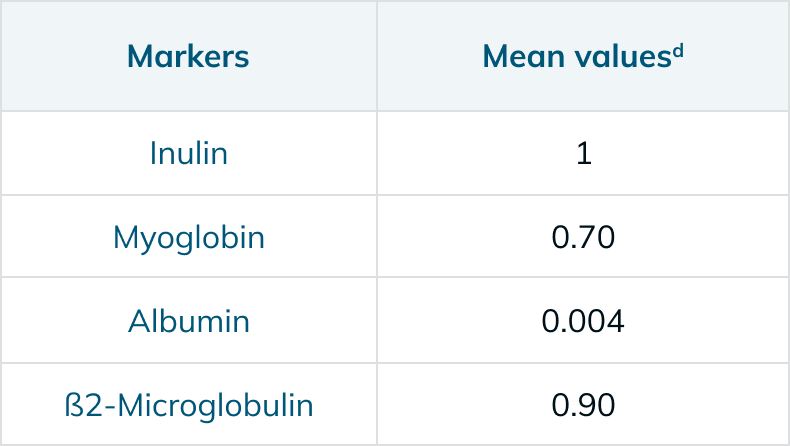
Performance Information5,6,13,†
a In vitro clearance: QB – 300 mL/min, QF – 10 mL/min, QD – 500 mL/min
b UF coefficient: QB – 300 mL/min, bovine blood Hct – 32 ± 3 %, protein – 60 ± 5 g/l
Discover our chronic hemodialysis portfolio
Dialyzers, advanced therapies and liquid hemodialysis concentrates that cater to the diverse needs of dialysis patients.
Additional resources
Products may not be available in all countries. If you have a question regarding product availability, please email us or complete this form.
†The measurements in the charts above are taken in accordance with EN ISO 8637-1. The values indicated are to be considered approximate and may vary due to measurement methods, inherent variations of the membrane, manufacturing and storage conditions. During the treatment, performance on the individual patient may vary due to variable clinical parameters of the patient.
The Clearum™ HS dialysers are non-active, non-invasive, class IIb medical devices, CE0123, manufactured by Bellco S.r.l.
Please refer to the device’s Instructions for Use for complete instructions, contraindications, warnings and precautions.
- Maduell F, Broseta JJ, Guillen MA, et al. Efficacy and Safety of the Clearum™ Dialyzer. Artif Organs. 2021;45(10):1195 1201.
- Keoleian G, Miller S, De Kleine R, Fang A, Mosley J. Life Cycle Material Data Update for GREET Model. University of Michigan: Ann Arbor. 2012:1-74.
- Efficiency of recovery for solvent and water in Clearum™. GmbH, (Sept 2nd-2019).
- GIPA-IIA, 2017, A comparison of gamma, e beam, x ray and ethylene oxide technologies for the industrial sterilization of medical devices and healthcare product.
- TR_SIE_007_R02_Clearum™ Sieving Coefficient Test Report.
- TR_CLE_008_R02_Clearum™ Clearance Test Report.
- Clearum™ HS Series LCD Report_Rev00.
- INR.086_R00 BPA Absence evaluation.
- Krieter DH, Canaud B, Lemke HD, et al. Bisphenol A in chronic kidney disease. Artif Organs. 2013;37(3):283-90.
- Müller TF, Seitz M, Eckle I, et al. Biocompatibility Differences with Respect to the Dialyzer Sterilization Method. Nephron. 1998;78(2):139-142.
- De S, Roy A. Hemodialysis Membranes: For Engineers to Medical Practitioners (1st Ed.). 2017. CRC Press.
- Allard B, Bergi R, Potier J, Coupel S. Dialyzers biocompatibility and efficiency determinants of sterilization method choice. Pharmacien Hospitalier et Clinicien. 2013;48(4):294-255.
- TR_UFR_008_R01_Clearum™ Ultrafiltration Rate Test Report.
- TR_SUW_003_R00_Clearum™ Shipping Unit Weight Test Report.
