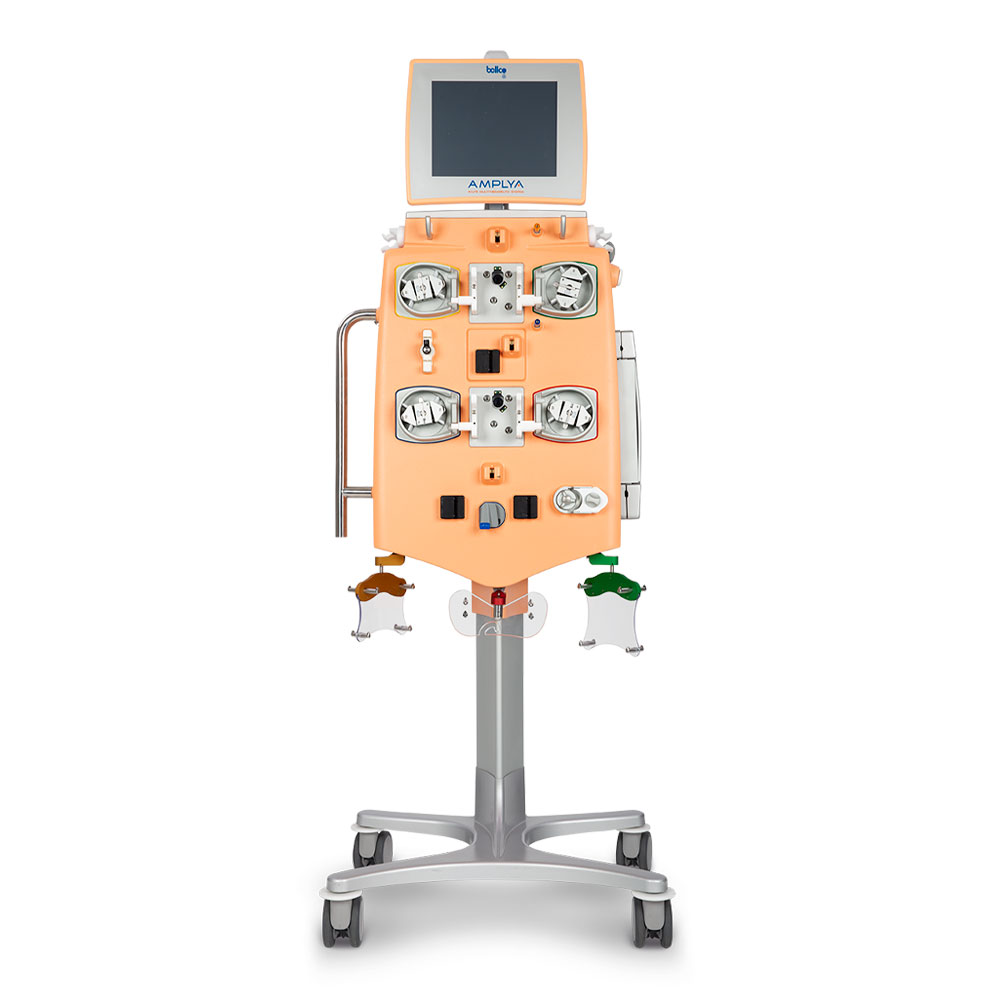
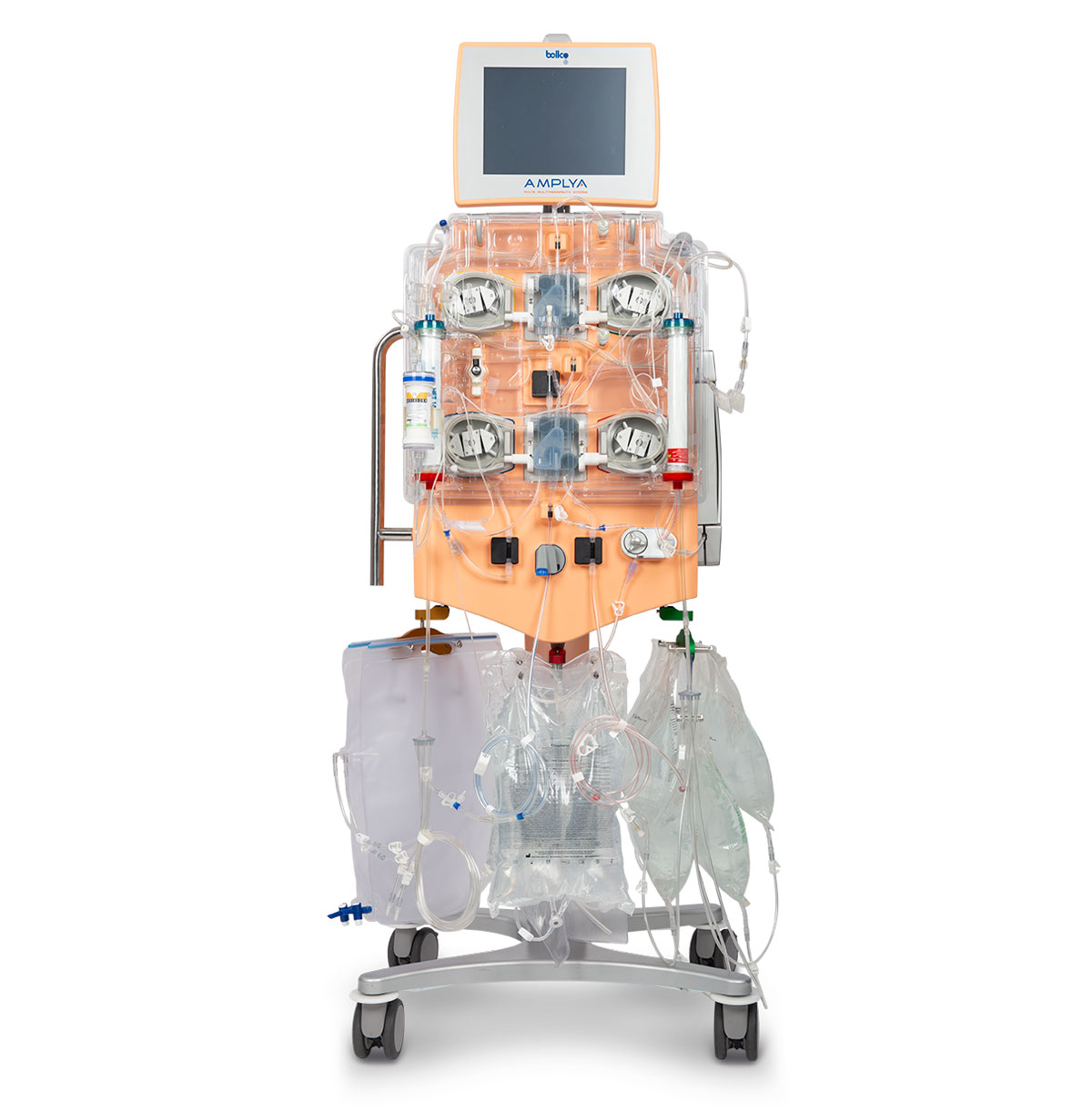
AMPLYA Acute Multitherapeutic system™
Delivers treatments involving continuous renal replacement therapy (CRRT), intermittent renal replacement therapy (IRRT), plasma adsorption (CPFA), plasmapheresis with reinfusion of new plasma (PEX) and haemoperfusion (HP) in clinical conditions of possible acute or chronic renal failure and water overload.
Indicated for patients weighing 15kg or more.
The plug and play platform for acute extracorporeal blood therapies
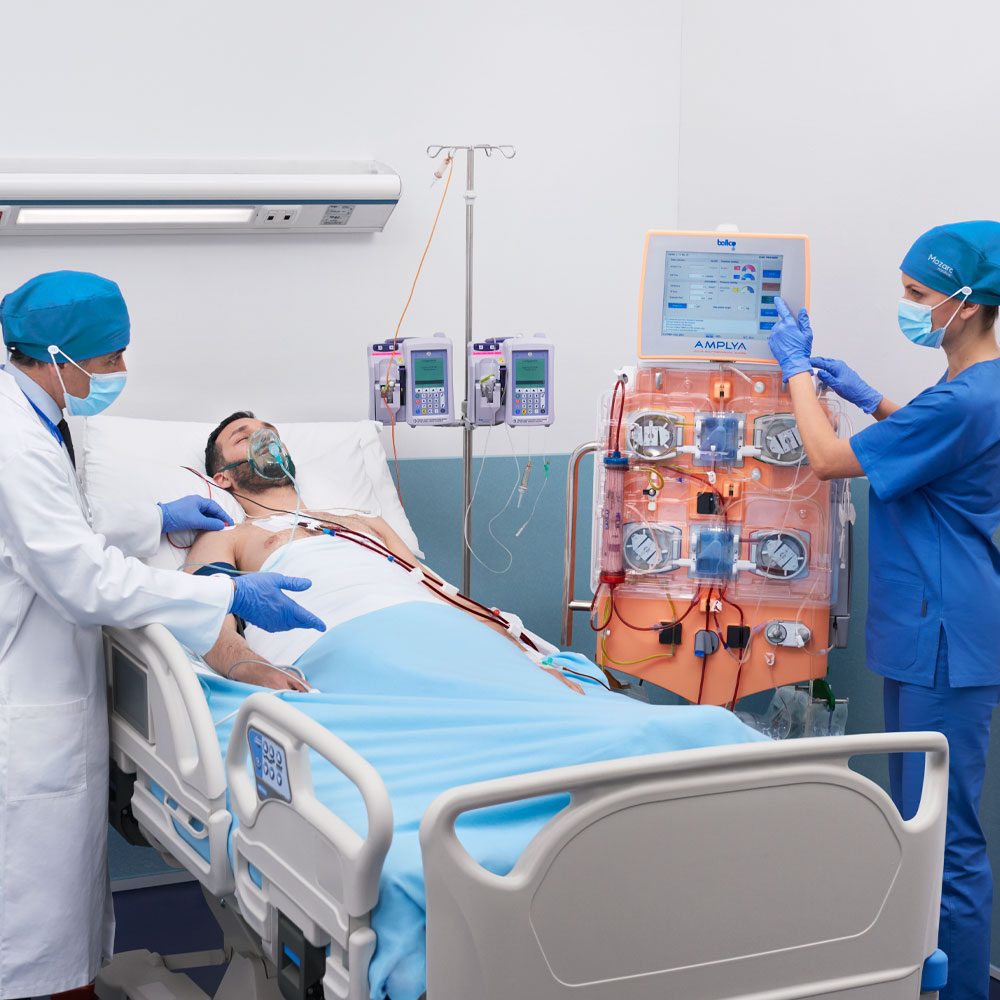
In treating acute renal failure, every minute counts. The AMPLYA Acute Multitherapeutic system™ offers a broad range of extracorporeal blood treatment therapies. With the AMPLYA system™, you have the option to change RRT (Renal Replacement Therapy) modality using the same circuit, saving you precious time1 when it matters most.
Renal replacement therapies
The AMPLYA system™ is able to perform a broad range of renal replacement therapies: continuous (CVVH, CVVHDF, CVVHD, SCUF) and intermittent (IHF, IHFD, IHD).2
Explore the AMPLYA system™
Treatments dedicated to complex patients
When a critically ill patient requires more than one extracorporeal procedure, options include3:
- Performing procedures sequentially
- Pausing one therapy to perform the other
- Performing the procedures simultaneously via separate vascular access
- Performing the procedures simultaneously via a single access but a combined circuit, either in series or in parallel
The AMPLYA system™ performs2:
- CPFA for patients with sepsis and multiple organ dysfunction
- PEX for the purpose of plasma clearance in critically ill patients
- HP for the purpose of blood clearance in critically ill patients

Anticoagulation
The international KDIGO (Kidney Disease Improving Global Outcomes) guidelines for anticoagulation in CRRT suggests using RCA (Regional Citrate Anticoagulation) rather than heparin in patients who do not have contraindications for citrate4
We offer 2 types of citrate concentration: 10/2 and 20/4.
The assisted citrate modality available on the AMPLYA system™ helps operators manage the treatment protocol without tables, providing suggested settings based on treatment type and monitoring.2
Additional resources
Products may not be available in all countries. If you have a question regarding product availability, please email us or complete this form.
The AMPLYA systemTM is an active, non-invasive, class IIb medical device CE0123 manufactured by Bellco S.r.l.
The pre-assembled devices for RRT and CPFA for Amplya are non-active, non-invasive class IIb medical devices CE0123 manufactured by Bellco S.r.l.
The Mediasorb Cartridge is a non-active, non-invasive class IIb medical device CE0123 manufactured by Bellco S.r.l.
The pre-assembled device for CPFA for Amplya and the Mediasorb Cartridge are included in KABL14P05 –KIT CPFA X AMPLYA procedure pack.
The pre-assembled device for PEX for Amplya is a non-active, non-invasive, class IIb medical device CE0123 manufactured by Bellco S.r.l.
The pre-assembled device for PEX for Amplya and the 5-litre drainage bag are included in KABLP05 –KIT PEX X AMPLYA procedure pack.
The pre-assembled device for HP for Amplya is a non-active, non-invasive, class IIa medical device CE0123 manufactured by Bellco S.r.l.
The 5-litre drainage bag (IB0514110) and infusion set for 5th pump on Amplya are non-active, non-invasive, class Is medical device CE0123 manufactured by Bellco S.r.l.
The infusion set for syringe pump is a non-active, non-invasive, class IIa medical device CE0123 manufactured by Bellco S.r.l.
The 5-litre (IB0507006) and 13-litre (IB0507008) waste bags are non-active, non-invasive, class I CE medical devices manufactured by Bellco S.r.l.
The bicompartmental bag HMB32, HMB34, HMB36 are non-active, non-invasive, class IIb medical devices CE0123 manufactured by Haemopharm Biofluids S.r.l.
The Citrachoice24 and Mixsol are medical devices manufactured by Paolo Gobbi Frattini S.r.l.
Please refer to the devices and procedure pack Instructions for Use for complete instructions, contraindications, warnings and precautions.
- Based on internal report BTR 208.
- Bellco S.r.l. (2022). AMPLYA system™ Operator’s Manual. IB5328069ENG Edition 2022-06 SW 5.7.
- Sanchez P, Ward D., Cunard R. Therapeutic plasma exchange in the intensive care unit: rationale, special considerations, and techniques for combined circuits. Ther Apher Dial. 2022;26(Suppl.1):41–52.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1-138.
